CBR4, also known as carbon tetrabromide, is a chemical compound that consists of one carbon atom and four bromine atoms. When determining whether CBR4 is a covalent or ionic compound, it is important to consider the electronegativity difference between carbon and bromine, as well as the nature of the bond formed between them. To understand the nature of the bond in CBR4, we need to examine the electronegativity values of carbon and bromine. Electronegativity is a measure of an atoms ability to attract electrons in a chemical bond. In general, when the electronegativity difference between two atoms is large, the bond is considered ionic. When the electronegativity difference is small, the bond is considered covalent. The electronegativity of carbon is 2.55, while the electronegativity of bromine is 2.96. The difference between these values is 0.41, which is relatively small. Based on this information, we can conclude that the bond formed between carbon and bromine in CBR4 is primarily covalent. In a covalent bond, atoms share electrons to achieve a stable electron configuration. In the case of CBR4, carbon shares one electron with each of the four bromine atoms, resulting in a stable configuration for both carbon and bromine. This sharing of electrons allows both carbon and bromine to achieve a complete outer shell of electrons, fulfilling the octet rule. It is worth noting that while the bond in CBR4 is primarily covalent, there may be some ionic character due to the difference in electronegativity. This means that there might be a slight transfer of electron density from carbon to bromine, resulting in a partial positive charge on carbon and a partial negative charge on bromine. However, this ionic character is relatively weak compared to compounds with larger electronegativity differences. In summary, CBR4, or carbon tetrabromide, is primarily a covalent compound. The bond between carbon and bromine is formed by the sharing of electrons, allowing both atoms to achieve a stable electron configuration. While there may be some ionic character due to the difference in electronegativity, it is relatively weak.
Is CBr4 (Carbon tetrabromide) Ionic or Covalent/Molecular?. To tell if CBr4 (Carbon tetrabromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and Br is a non-metal. When we have a. cbr4 covalent or ionic. Carbon tetrabromide - Wikipedia. Tetrabromomethane, CBr 4, also known as carbon tetrabromide, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature cbr4 covalent or ionic. Physical properties Tetrabromomethane has two polymorphs: crystalline II or β below 46.9 °C (320.0 K) and crystalline I or α above 46.9 °C.. Solved 1 cbr4 covalent or ionic. Classify the compound CBr4 as ionic or covalent. - Chegg. Expert Answer 1. CBr4 covalent name of the compound - carbo … View the full answer Transcribed image text: 1. Classify the compound CBr4 as ionic or covalent cbr4 covalent or ionic. covalent What is the name of this compound? Carbon tetrabromide 2. Classify the compound Cr2S3 as ionic or covalent? ionic What is the name of this compound? Chromium (III) sulfide < 3.. Is CBr4 Ionic or Covalent? (And Why?) - Knords Learning. Is CBr4 Ionic or Covalent? (And Why?) May 27, 2023 by Jay Rana CBr4 is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, C is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound.. Carbon tetrabromide | CBr4 | CID 11205 - PubChem cbr4 covalent or ionic. Carbon tetrabromide | CBr4 | CID 11205 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . cbr4 covalent or ionic
i want to fuck all hot girls
. Covalent & Ionic Compounds Ionic, Metal or Non Metal? Click the card to flip 👆 Metal Click the card to flip 👆 1 / 39 Flashcards Learn Test Match Created by Crystani_CMickey Terms in this set (39) Ionic, Metal or Non Metal? Metal Covalent, Metal or Non Metal? Non-Metal Ionic, Shared or Not Sharred? E- Not Shared Covalent, Sharred or Not Sharred?. Chem Homework 3 Flashcards | Quizlet. Classify these compounds as ionic or covalent: KCl Ca (NO3)2 CBr4 AlBr3 Br2 NO2 NH3 NaNO2 KCl: ionic Ca (NO3)2: Ionic. Is the compound CBr4 ionic or covalent? Explainsydney anglican sex abuse defrock
. - Homework.Study.com. Is the compound CBr4 C B r 4 ionic or covalent? Explain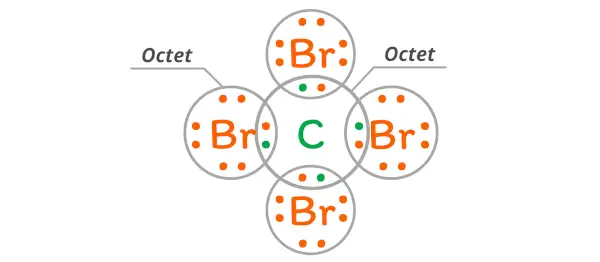
entrepreneurs are gamblers
cash sweepstakes and giveaways
. Is CBr4 an ionic or covalent bond - Bengis Lifekamo fitness coupon
. Answer: CBr4 ( Carbon tetrabromide ) is a covalent bond What is chemical bond, ionic bond, covalent bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.. Is CBr4 Polar or Nonpolar? - Techiescientistwheel of fortune list of sweepstakes winners
. Carbon tetrabromide is a chemical compound with its chemical formula as CBr4. its systematic IUPAC name is Tetrabromomethane. It is a solvent for a wide range . The covalent and ionic are the strongest among all these bond forces cbr4 covalent or ioniccraigslist dogs phoenix az
. The covalent bonds can be polar and nonpolar depending upon various factors like electronegativity, geometrical . cbr4 covalent or ionic. Is CBr4 Polar or Nonpolar? (And Why?) - Knords Learning. CBr4 is a NONPOLAR molecule. . The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the two atoms cbr4 covalent or ionic. Have a look at the above image. If the electronegativity difference , then the bond is nonpolar covalent bond. If the electronegativity difference , .. 5.10: Electronegativity and Bond Polarity - Chemistry LibreTexts cbr4 covalent or ionic. Figure 5.10. 1: Electronegativities of the Elements. Electronegativities are used to determine the polarity of covalent bonds. The polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized in the following table: Electronegativity Difference.. CBr4 Lewis Structure - How to Draw the Dot Structure for the Carbon .i win iphone x
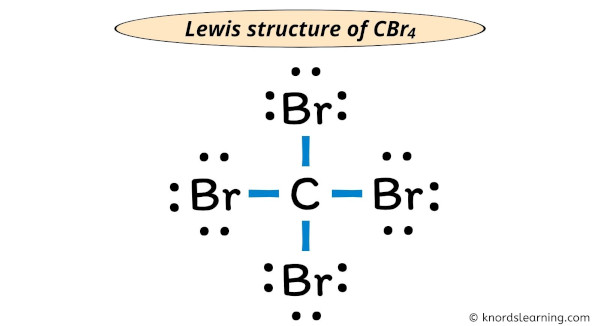
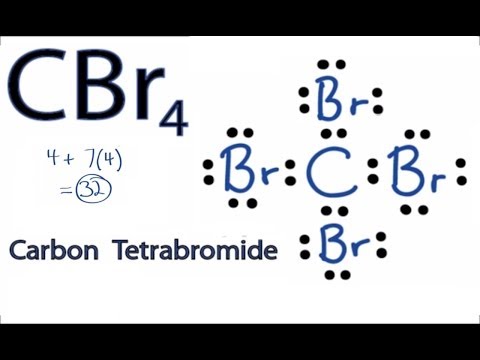
. Transcript: This is the CBr4 Lewis structure: Carbon Tetrabromide cbr4 covalent or ionic. Carbon is in group 4 or 14, so it has 4 valence electrons. Bromine in group 7 or 17, so it has 7, and we have 4 Bromines. So 4 plus 28 equals 32 total valence electrons. Carbon, thats the least electronegative, thatll go in the center; and on the outside well put the Bromine .